Case Details
Lesion Characteristics
Lesion Size (diameter): 25 mm
Lesion Location: Right Upper Lobe
Bronchus Sign: Yes
Visible on Fluoro: Yes
REBUS Verification: Concentric
Case Information
Full Procedure Time: 75 minutes (this included biopsy of 25mm nodule in right upper lobe (RUL) as well as a biopsy of a right lower lobe (RLL) nodule and staging of lymph nodes)
ROSE: Adenocarcinoma from needle biopsy and touch preps from transbronchial lung biopsy (TBBx)
Final Pathology Report: Well-differentiated adenocarcinoma of the lung from needle biopsies and transbronchial lung biopsies
Background
An 88-year-old woman with no smoking history but with a remote history of breast cancer was referred with a solitary pulmonary nodule in the right upper lobe (RUL). The patient had two previous attempts to biopsy from the RUL lesion prior to the referral but both attempts proved non-diagnostic. A Fluorodeoxyglucose (FDG) PET scan of the RUL lesion exhibited a standardized uptake value (SUV) of 4.9. At this point, the patient was referred to Dr. D. Kyle Hogarth at the University of Chicago Medical Center for a second opinion. The patient returned a normal pulmonary function test (PFT). A robotic-assisted navigation bronchoscopy procedure utilizing the Body Vision system for real-time, intraoperative 3D imaging in conjunction with the Auris MONARCH® platform was scheduled.
The Procedure
Planning
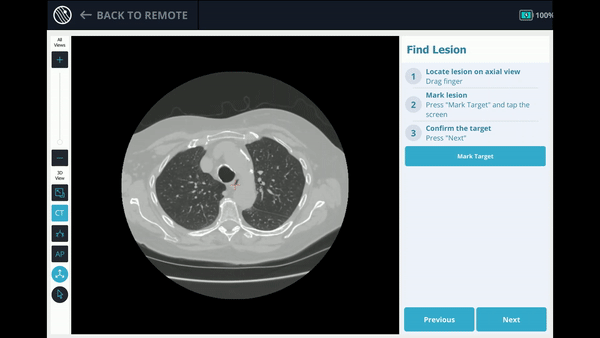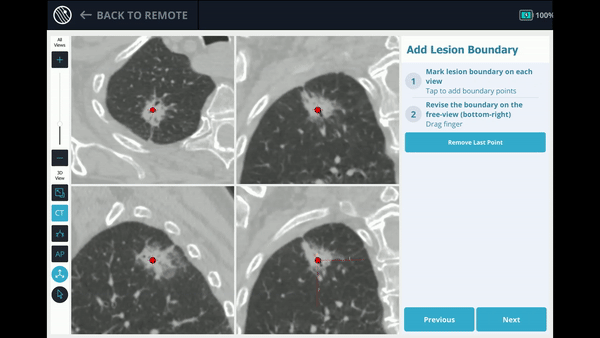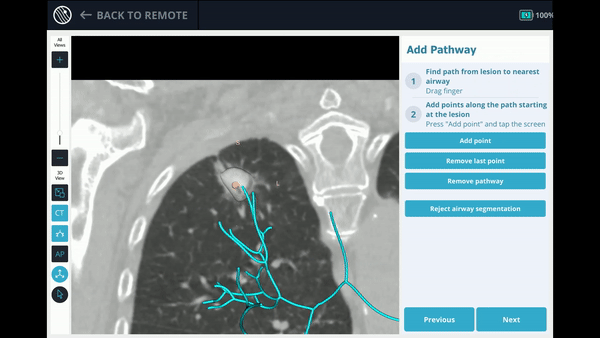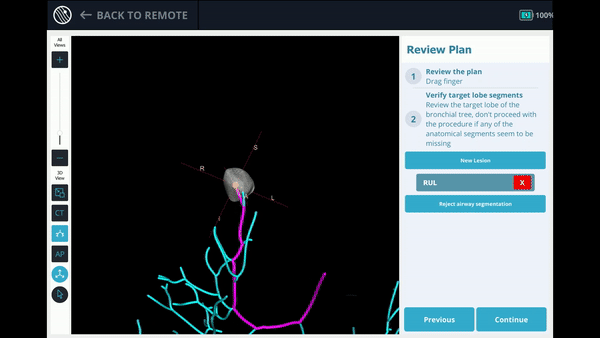
The Body Vision system derives the airways from the pre-operative CT as well as uses the physician’s marking of the lesion and lesion boundaries to approximate lesion location prior to the procedure. During the procedure, lesion location and positioning of the bronchoscope and tools relative to the lesion is provided by real-time, intraoperative 3D imaging.
CABT™ Registration
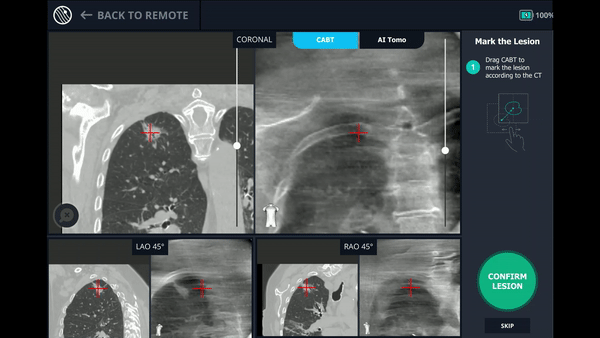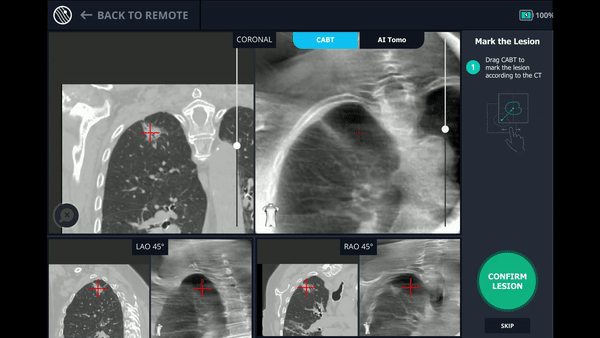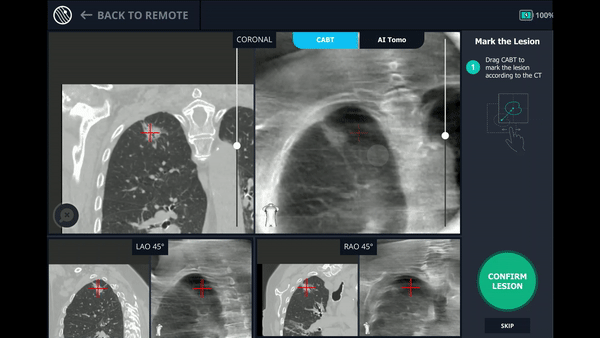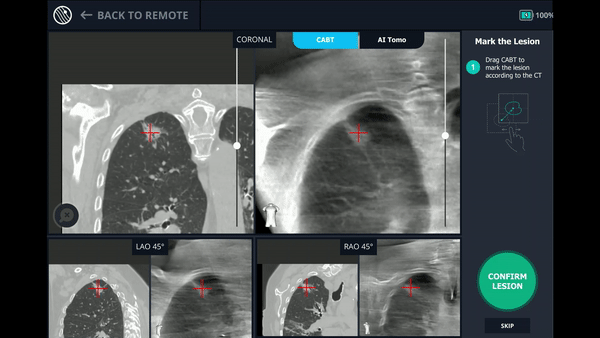
Using any conventional C-arm, Body Vision's C-arm-based computational tomography (CABT) technology provides intraoperative 3D images. Registration of the Body Vision system entails a CABT scan of the main carina and a second CABT scan iso-centered around the lesion which provides visualization of the actual lesion and lesion location, even of radiolucent lesions which are typically not fluoro visible.
Navigation
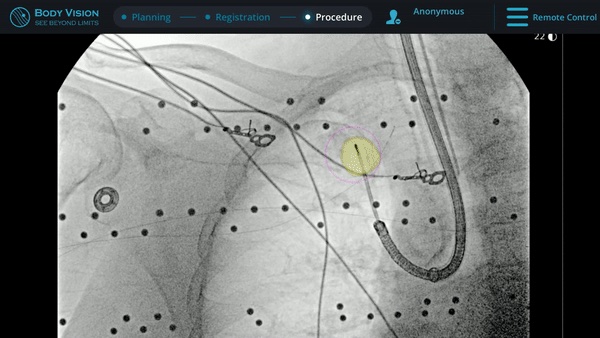
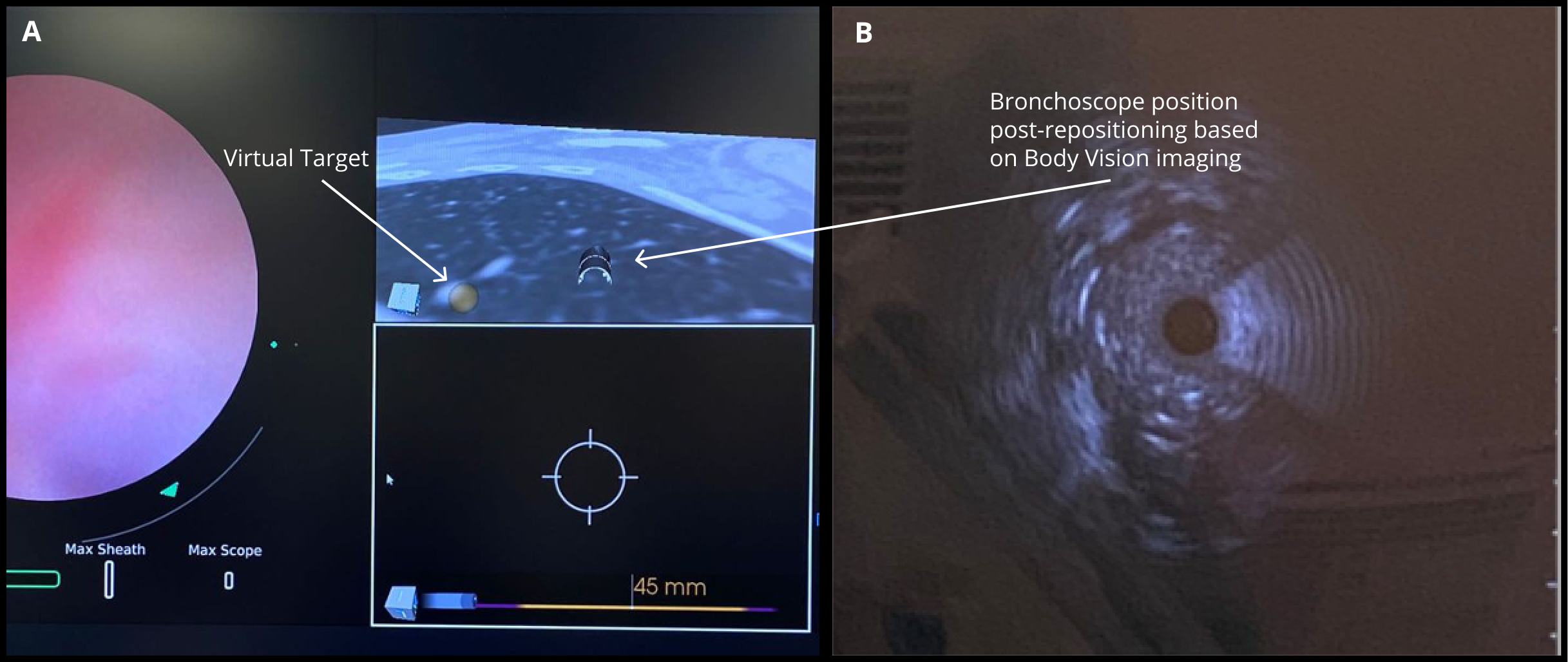 The MONARCH® platform was initialized and used to navigate to within close proximity of the virtual target using the robotic platform’s electromagnetic navigation. The C-arm was then brought into position over the patient and a CABT scan with the Body Vision was performed to determine true lesion location (A).
The MONARCH® platform was initialized and used to navigate to within close proximity of the virtual target using the robotic platform’s electromagnetic navigation. The C-arm was then brought into position over the patient and a CABT scan with the Body Vision was performed to determine true lesion location (A).
The robotic bronchoscope was repositioned based on the new information provided by Body Vision’s intraoperative 3D imaging and lesion’s presence at the location as imaged by Body Vision was confirmed with Radial Endobronchial Ultrasound (REBUS). REBUS returned a concentric view (B). Because Body Vision’s intraoperative 3D imaging is captured in real-time when the patient is on the table and is not reliant on pre-operative CT images, CT-to-body divergence is eliminated and, as in this case, accurate navigation to the actual lesion was enabled.
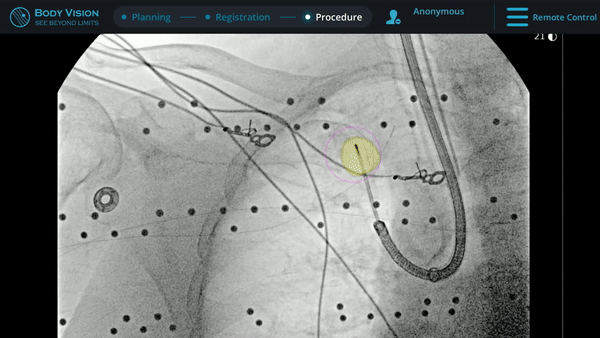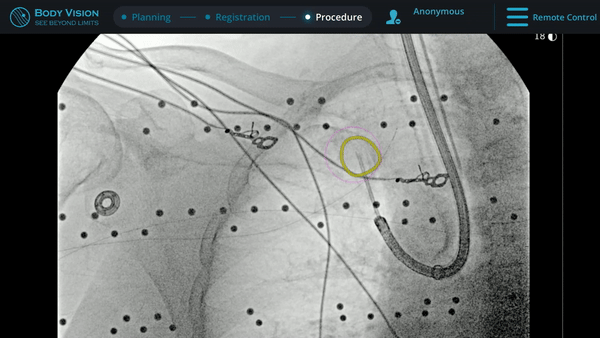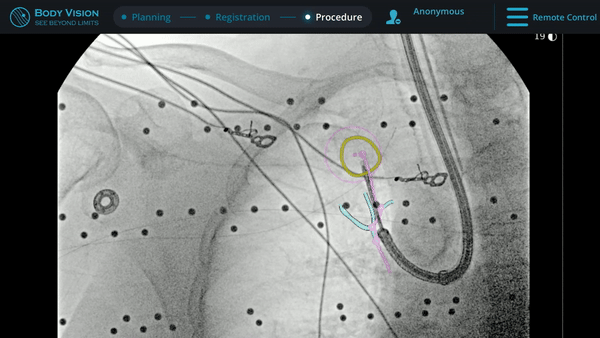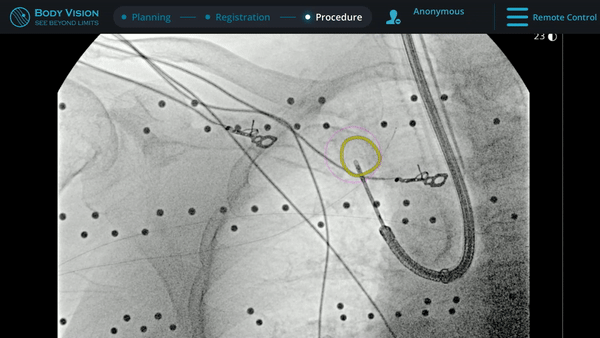
Tool-In-Lesion Confirmation
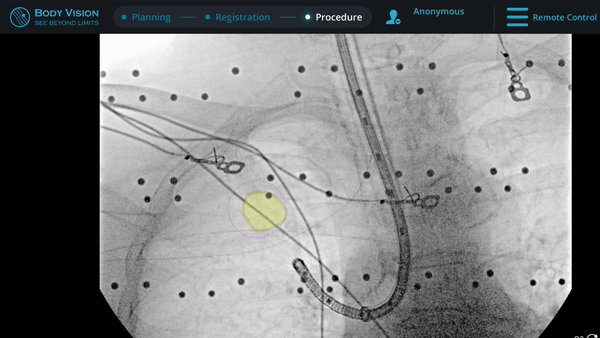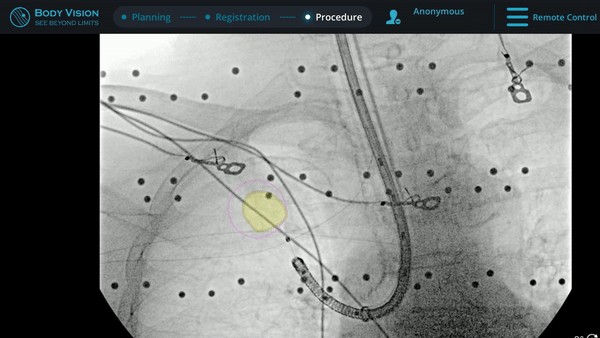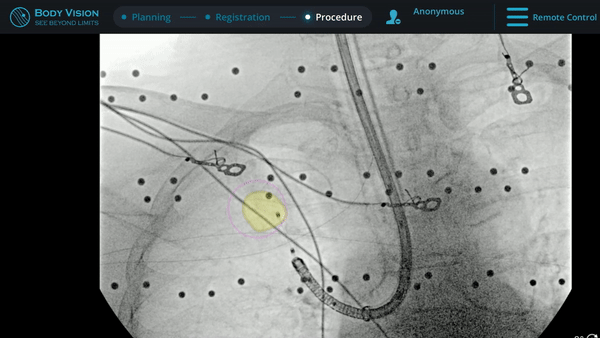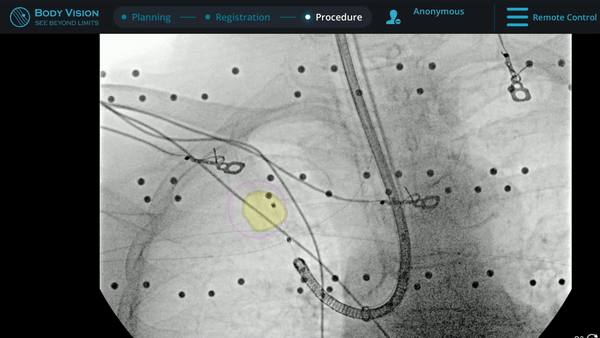
Under Body Vision’s augmented fluoroscopy where the C-arm fluoro image is augmented with actual lesion location provided by the most recent CABT scan, Dr. Hogarth was able to see in real-time as he introduced a Body Vision stylet (no longer needed for robotic bronchoscopy cases) with radiopaque markers down the working channel of the MONARCH and guides it to the lesion.
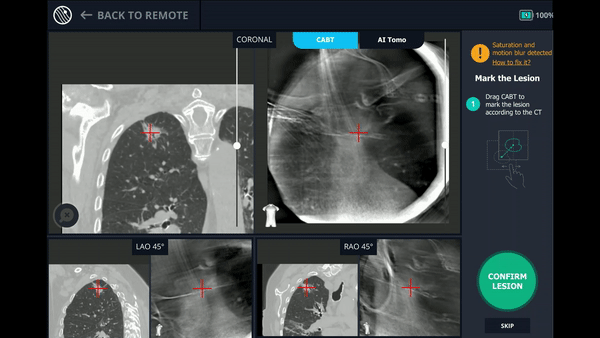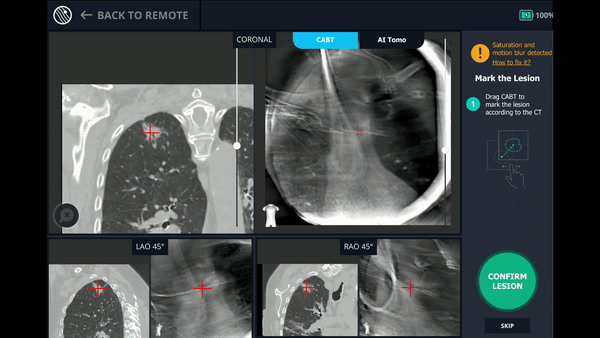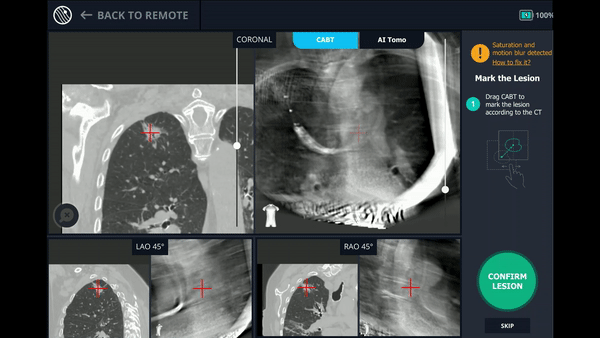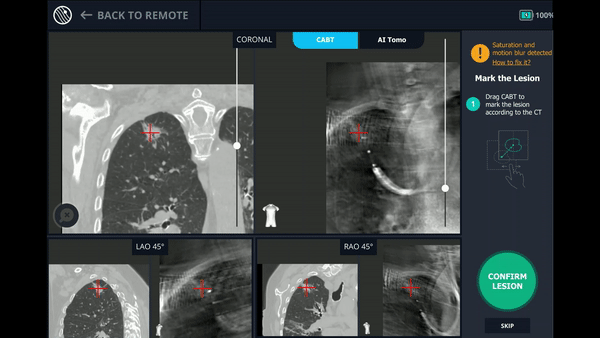
With the Body Vision stylet in place, a CABT was performed to provide visual confirmation of tool-in-lesion in multiple 3D planes.
Biopsy
With tool-in-lesion confirmed under Body Vision’s intraoperative 3D imaging, the Body Vision stylet was retracted and a biopsy needle was introduced down the working channel of the MONARCH. Under augmented fluoroscopy which showed both lesion boundaries and biopsy tool location in real-time, Dr. Hogarth was able to acquire biopsy samples and ensure that those samples were from within the lesion. Rapid On-Site Evaluation (ROSE) assessed that samples collected via needle biopsy and touch preps from transbronchial lung biopsy (TBBx) were adequate and consistent with adenocarcinoma. Endobronchial Ultrasound (EBUS) staging of the mediastinal (station 1) and hilar (station 10) nodes radiographically showed the lymph nodes were of normal size. The diagnosis of well-defined adenocarcinoma was confirmed via the final pathology report.
Conclusion
This use case demonstrates the value of Body Vision’s intraoperative 3D imaging regardless of bronchoscopy platform. The stability and articulation provided by robotic bronchoscopy platforms provide a clear clinical value during navigation bronchoscopy. However, without real-time intraoperative imaging like that provided by Body Vision, the current generation of robotic bronchoscopy platforms cannot account for CT-to-body divergence. Utilizing the Body Vision intraoperative 3D imaging system in concert with the Auris MONARCH robotic bronchoscopy platform, Dr. Hogarth was able to definitively diagnose a peripheral 2.5cm RUL lesion.
About Dr. Hogarth

Kyle Hogarth, MD, FCCP
Professor of Medicine
Director of Bronchoscopy, Co-Director of Lung Cancer Screening Program
University of Chicago Medical Center